CDSCO expert panel to review covid vaccine applications on Wednesday
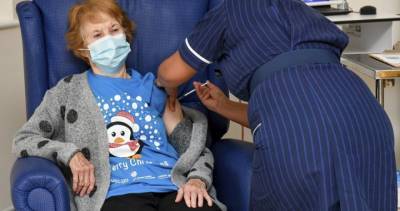
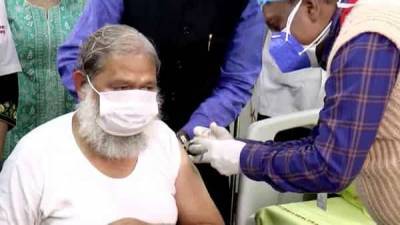
NEW DELHI : An expert committee of the Central Drugs Standard Control Organisation (CDSCO) will meet on Wednesday to review applications of Pfizer, Serum Institute of India and Bharat Biotech seeking emergency use authorisation for their COVID-19 vaccine candidates, official sources said on Monday night.The decision was taken late on Monday evening after the Hyderabad-based Bharat Biotech became the third pharmaceutical firm to apply to the Drugs Controller General of India (DCGI) for emergency use authorisation for its indigenously developed COVID-19 vaccine Covaxin.The Indian arm of US pharmaceutical giant Pfizer had on December 4 sought approval for its vaccine from the central drug regulator, after the firm secured such clearance in the.
Read more on livemint.com