Novavax launches pivotal U.S. trial of dark horse COVID-19 vaccine after manufacturing delays
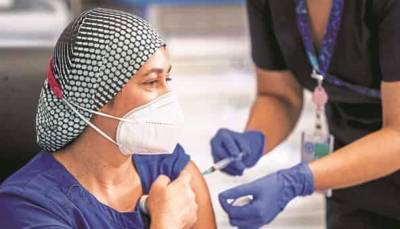
A volunteer receives a COVID-19 vaccine candidate or placebo in October in the phase III trial Novavax launched in the United Kingdom.
By Meredith WadmanScience's COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation.Novavax, a once-struggling biotech company that was rescued by $2 billion in funding for its promising COVID-19 vaccine candidate from the Coalition for Epidemic Preparedness Innovations and the U.S.
government, announced today the long-awaited start of its U.S. efficacy trial. Novavax has already fully enrolled an efficacy trial in the United Kingdom with more than 15,000 volunteers, whose data it plans to use to support its application for European regulatory approval.
Read more on sciencemag.org