WHO gives emergency approval to Chinese-made Sinovac vaccine
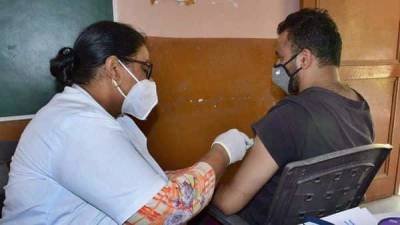
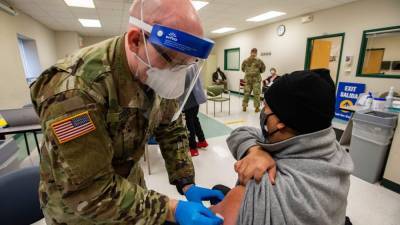
The World Health Organization has approved a Covid-19 vaccine made by drugmaker Sinovac Biotech for emergency use listing, paving the way for a second Chinese shot to be used in poor countries.
A WHO emergency listing is a signal to national regulators on a product's safety and efficacy. It will also allow the shot to be included in COVAX, the global programme to provide vaccines mainly for poor countries, which faces major supply problems due to an Indian export suspension.
The independent panel of experts said in a statement it recommended Sinovac's vaccine for adults over 18, with a second dose 2-4 weeks later.
There was no upper age limit as data suggested it is likely to have a protective effect in older people. The WHO's technical
Read more on rte.ie