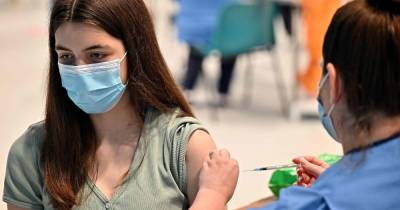
Bharat Biotech gets DCGI nod to conduct phase II/III trial of its nasal Covid vaccine
Bharat Biotech has received approval from India's drug regulator to conduct mid-to late-stage trials of their nasal Covid-19 vaccine candidate, the Union ministry of science said on Friday. “This is the first of its kind Covid-19 jab to undergo human clinical trials in India," the ministry said. " “BBV154 is an intranasal replication-deficient chimpanzee adenovirus SARS-CoV-2 vectored vaccine.BBIL has in-licensed technology from Washington University in St Louis, USA," it added.
Early stage trials of the vaccine candidate, BBV154, has been completed in subjects aged 18 to 60 years. The company has said that the doses of the vaccine administered to healthy volunteers were found to be well tolerated. “Bharat Biotech’s BBV154 Covid
Read more on livemint.com