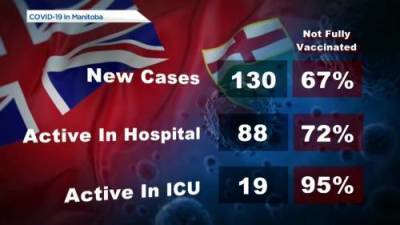
FDA delays decision on Moderna COVID-19 vaccine for kids 12-17
CAMBRIDGE, Mass. - A decision on the authorization of Moderna’s COVID-19 vaccine for kids ages 12 to 17 has been delayed while U.S.
regulators continue to study the rare risk of heart inflammation, the company said Sunday. The U.S. Food and Drug Administration told Moderna on Friday evening that its review could last until January, according to a company statement.
Moderna also said it will delay filing a request for emergency-use authorization of a lower dose of the vaccine for 6- to 11-year-olds."The safety of vaccine recipients is of paramount importance to Moderna," the company said in the statement.
Read more on fox29.com