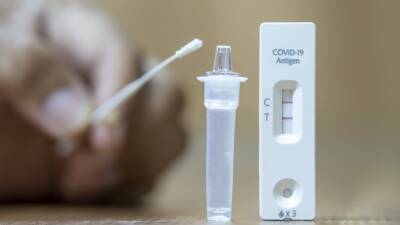
FDA recalls 2.2 million at-home coronavirus tests due to false positives
WASHINGTON - The FDA announced this week that the recall of more than 2.2 million at-home COVID-19 tests by digital diagnostics firm Ellume is being classified as a class 1 recall, which is the most serious type due to the potential for "serious adverse health consequences or death."Ellume's at-home test detects proteins from the SARS-CoV-2 virus through a less invasive nasal swab than the one that health providers normally use.
The recall is due to a "manufacturing issue" that was first identified by the company last month and could lead to a false-positive result.
The FDA said Wednesday that it has received 35 reports of false-positive test results, but noted that negative test results are still reliable and not affected by the.
Read more on fox29.com