India sees rise in Covid-19 case with 16,047 infections; recovery rate at 98.54%
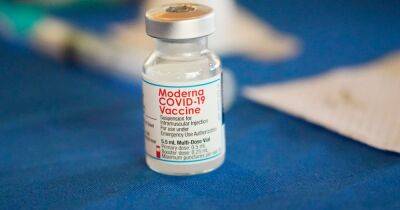
official sources informed that the Union health ministry is likely to approve Corbevax as the third dose for for people above 18 years.
In case the vaccine is approved for the third dose, this would be India's first where the booster dose is different from the primary vaccination.
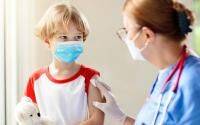
The Drugs Controller General of India (DCGI) on June 4 approved Corbevax as a precaution dose for those aged 18 and above. The Corbevax vaccine is currently being administered among children in the age group of 12 to 14 years.
Meanwhile, the national capital on Tuesday reported 2,495 new cases and seven deaths, as per Delhi health bulletin. The positivity rate in the national capital stood at 15.41% and active cases were at 8,506, the Delhi health bulletin notified on Tuesday.
Read more on livemint.com