Trial: Metformin, ivermectin, fluvoxamine don't prevent severe COVID
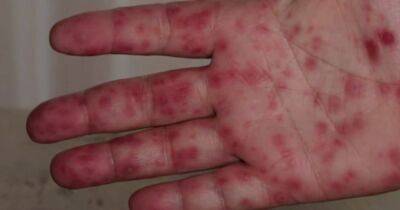
A phase 3 randomized, controlled trial published today in the New England Journal of Medicine shows that three drugs repurposed for the treatment of COVID-19—metformin, ivermectin, and fluvoxamine—didn't prevent hypoxemia, an emergency department (ED) visit, hospitalization, or infection-related death, although a secondary analysis finds that metformin may hold some promise.The double-blind COVID-OUT Trial Team, led by University of Minnesota researchers, evaluated the outcomes of 1,323 overweight or obese (body mass index [BMI], 25 kilograms per meter squared or greater) adult COVID-19 outpatients enrolled within 3 days of diagnosis and less than 7 days after symptom onset.Median patient age was 46 years, 56% were women (6% of whom were pregnant), and 52.2% had been vaccinated against COVID-19.
Patients were recruited from six US healthcare systems in Minnesota, Illinois, California, Colorado, and Indiana and enrolled from Dec 30, 2020, to Jan 28, 2022, with follow-up to Feb 14.Metformin is a drug used to treat diabetes, ivermectin is used to treat parasite infestations, and fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) used as therapy for obsessive-compulsive disorder and other conditions, such as depression.Patients were randomly assigned to one of six groups to receive one of the three drugs individually, a combination of either metformin and fluvoxamine or metformin and ivermectin, or a placebo.
Participants received two types of pills for 3 to 14 days to keep them from knowing which treatment they were receiving. They documented their symptoms each day and completed a survey after treatment ended.Secondary analysis shows promise but isn't definitiveHypoxemia, ED visit, hospitalization, or death
Read more on cidrap.umn.edu