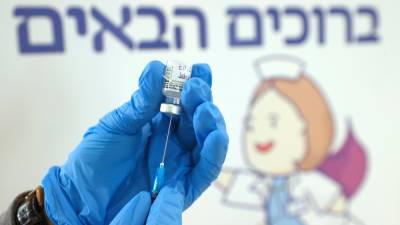
Pfizer/BioNtech to seek approval for third jab
Pfizer and BioNtech have announced that it will seek regulatory approval for a third dose of their Covid-19 vaccine. The pharmaceutical companies said that, based on drop-offs in efficacy seen in Israel after six months, they believe that a third dose may be needed within six to 12 months after full vaccination. "While protection against severe disease remained high across the full 6 months, a decline in efficacy against symptomatic disease over time and the continued emergence of variants are expected," a statement from the two companies said.
It comes after initial data from a trial showed a third shot pushed antibody levels five to 10 times higher against the original strain and the Beta variant, compared to the first two doses alone,
Read more on rte.ie