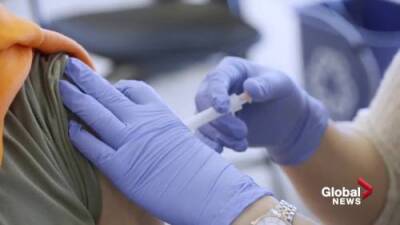
Zydus Cadila gets DCGI nod to initiate Phase-3 clinical trials for covid-19 vaccine
NEW DELHI : Drug firm Zydus Cadila on Sunday said it has received DCGI approval to initiate Phase III clinical trials of its COVID-19 vaccine ZyCoV-D.The company will now be initiating Phase III clinical trial in around 30,000 volunteers, Zydus Cadila said in a statement.ZyCoV-D was found to be safe, well-tolerated and immunogenic in phase I and II clinical trials, it added.The phase II study of ZyCoV-D had been conducted in over 1,000 healthy adult volunteers as part of the adaptive Phase I/II dose-escalation, multi-centric, randomised, double-blind placebo-controlled study, the drug firm said.The trial has reviewed by an independent data safety monitoring board (DSMB) and reports were submitted to the Central Drugs Standard Control.
Read more on livemint.com