AstraZeneca secures orders for virus vaccine under testing
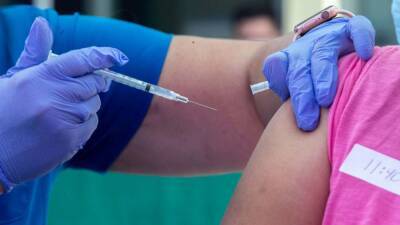
LONDON – Drug maker AstraZeneca said Thursday it had secured its first agreements for 400 million doses of a COVID-19 vaccine it is testing, bolstered by an investment from the U.S.
vaccine agency. The Anglo-Swedish company reported it had received more than $1 billion from the U.S. Biomedical Advanced Research and Development Authority for the development, production and delivery of the vaccine, starting this fall.
The investment will accelerate the development and production of the vaccine, AstraZeneca Chief Executive Pascal Soriot said.
It had already joined forces with the British government and is in discussions with the Serum Institute of India and other potential partners to increase production and distribution. “We will do
Read more on clickorlando.com