COVID-19 boosters likely to be expanded in coming weeks, US health officials predict
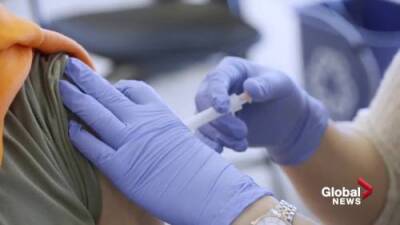
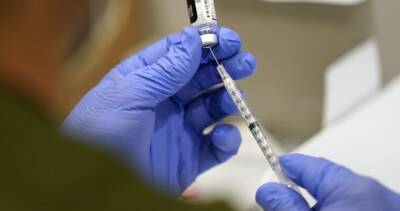
the panel’s recommendation Friday was correct based on a "snapshot" of available data on the effectiveness of Pfizer’s two-shot regimen over time.
But he said real-time data from the U.S. and Israel continue to come in showing waning efficacy among broader groups of people that will need to be addressed soon.Collins, who also appeared on CBS’ "Face the Nation," said: "I think there will be a decision in the coming weeks to extend boosters beyond the list that they approved on Friday."On Friday, the panel voted to endorse COVID-19 booster shots only for Americans 65 and over, and for those who are at high risk for severe disease. RELATED: FDA advisory panel OKs Pfizer COVID-19 booster for 65 and older, votes no for youngerDr.
Read more on fox29.com