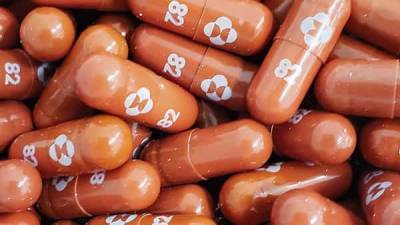
Covid treatment: Merck to seek emergency approval for pill that can be taken at home
Planned Analysis The latest findings came out of a previously planned analysis of 775 patients who had been in the clinical trial since at least early August.
About 7% of patients on molnupiravir went on to be hospitalized or die, versus 14% of those on a placebo. None of those taking molnupiravir died, compared with eight deaths among the placebo group.
Merck had planned to enroll 1,550 patients in the trial, with around 90% already signed up, according to a release. Patients who were taking a placebo will have the opportunity to start on molnupiravir, Davis said.
The company said in the statement that it expects to produce 10 million courses of treatment by year-end, with more expected to be produced in 2022.
Read more on livemint.com