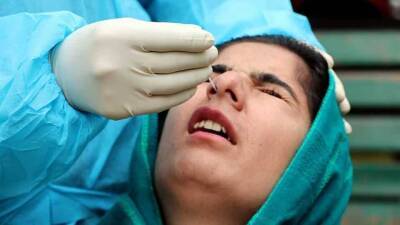
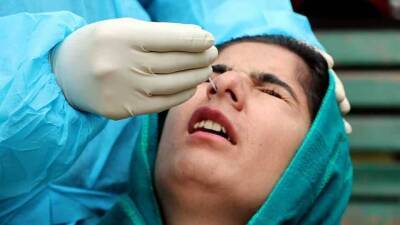
Data pertaining to Covid vaccine trials should be made public: Doctors' forum
Covid-19 vaccine trials should be made public to enable scientists and healthcare workers to make informed choices regarding administration of vaccination.In a statement issued on Sunday, the Progressive Medicos and Scientists Forum said the Clinical Trial (PMSF) Phase 3 data should be evaluated in a transparent manner as and when available for India for both vaccines and the decision to grant Emergency Use Authorization (EUA) may be revisited once the Phase 3 trials are completed.They demanded that all data pertaining to the vaccine trials should be made public to enable scientists and healthcare workers to make informed choices regarding administration of vaccines for themselves and for their larger communities."All healthcare workers.
Read more on livemint.com