FDA gives emergency OK to Eli Lilly antibody treatment for COVID-19
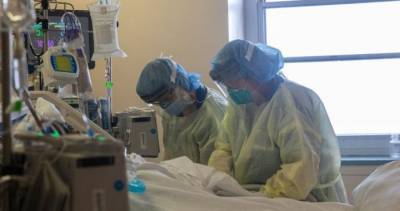
WASHINGTON – U.S. health officials have allowed emergency use of the first antibody drug to help the immune system fight COVID-19, an experimental approach against the virus that has killed more than 238,000 Americans.The Food and Drug Administration on Monday cleared the experimental drug from Eli Lilly for people 12 and older with mild or moderate COVID-19 not requiring hospitalization.
It's a one-time treatment given through an IV.The therapy is still undergoing additional testing to establish its safety and effectiveness.
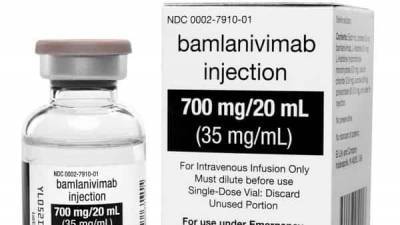
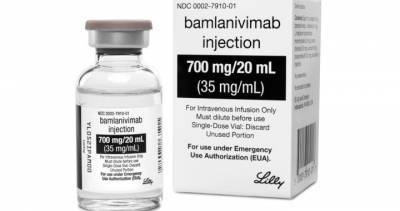
It is similar to a treatment President Donald Trump received after contracting the virus last month.Early results suggest the drug, called bamlanivimab, may help clear the coronavirus sooner and possibly cut.
Read more on clickorlando.com