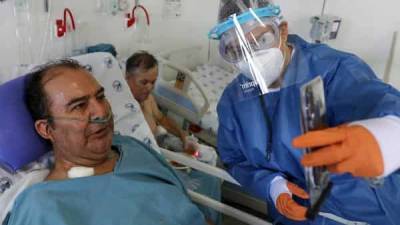
Hetero Labs gets DGCI nod to make Gilead's remdesivir for COVID-19 treatment
₹5,000 to 6,000 for a 100 milligram dose, Hetero said BENGALURU : India's drug regulator has given Hetero Labs the green light to manufacture and market its generic version of Gilead Science's experimental COVID-19 treatment remdesivir, the Indian pharmaceutical company said on Sunday.The drug, which will be marketed under the brand name Covifor, will likely be priced at ₹5,000 to 6,000 ($66-$79) for a 100 milligram dose, Hetero said.India's Cipla Ltd has also received approval from the Drug Controller General of India (DGCI) to manufacture and market the drug, according to a media report.
Cipla and DGCI were not immediately available for comment.Gilead Sciences Inc signed non-exclusive licensing pacts last month with five generic.
Read more on livemint.com