How to launch a drug in a pandemic with Immunomedics
New Jersey-based Immunomedics focuses on developing novel antibody drug conjugates (ADCs) for hard-to-treat cancers. At the end of April, its first ADC, Trodelvy (sacituzumab govitecan), received US Food and Drug Administration (FDA) accelerated approval in heavily pre-treated metastatic triple negative breast cancer (TNBC).
People in this patient group have essentially experienced disease progression despite being treated with multiple available chemotherapy regimens, creating a significant unmet need in this type of breast cancer, which disproportionately affects younger people and ethnic minorities.
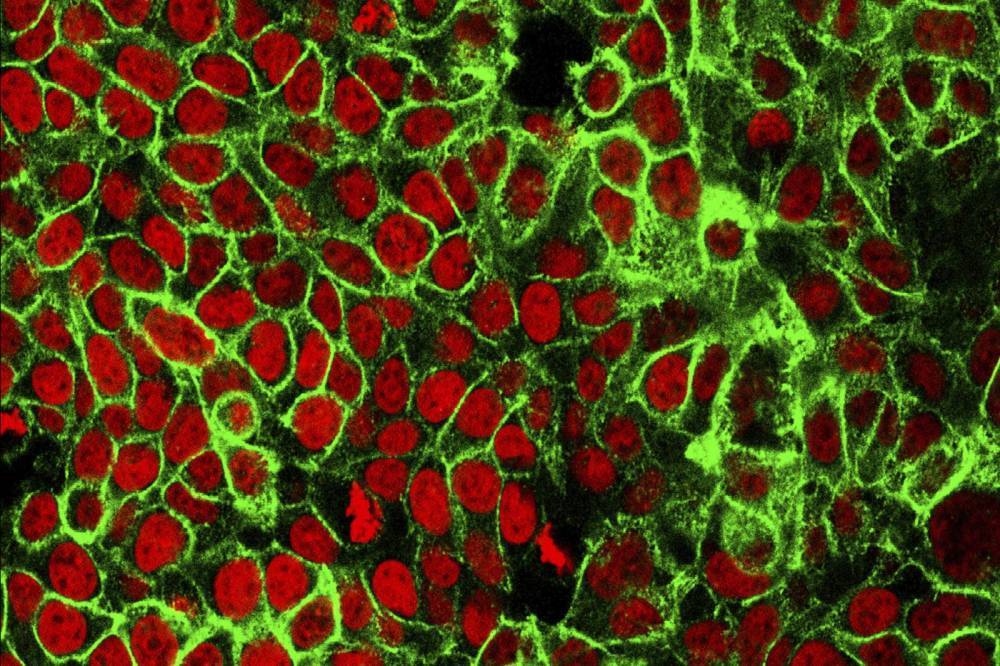
Trodelvy targets Trop-2, a glycoprotein highly expressed on the surface of TNBC cells. It is the first therapy directed at this target in
Read more on pharmaceutical-technology.com