Pfizer hopes to submit COVID-19 vaccine data for youngest kids in weeks
Signage outside Pfizer headquarters in New York, U.S., on Sunday, Nov. 7, 2021. Pfizer Inc. is aiming to submit data from its experimental Covid-19 pill to U.S.
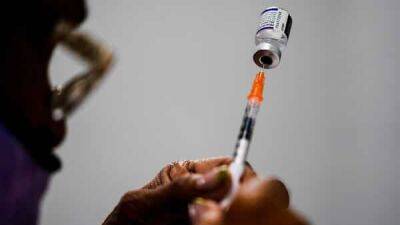
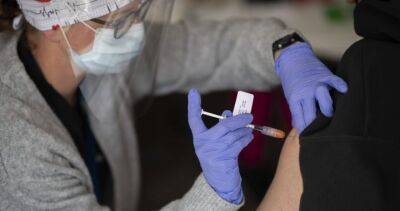
regulators by the Nov. 25 Thanksgiving holiday, potentially clearing the way for an emerg Pfizer now hopes to tell U.S. regulators how well its COVID-19 vaccine works in the littlest kids by late May or early June.Pfizer is testing three extra-small doses of its vaccine in children under 5 after two shots didn't prove quite strong enough.
Initial results had been expected last month but the company laid out the latest timeline Tuesday during its discussion of quarterly financial results.Currently in the U.S., only children ages 5 or older can be vaccinated, using Pfizer’s vaccine -- leaving 18 million younger tots unprotected.RELATED: 3 out of 4 US kids have had COVID-19, CDC researchers estimateMelissa Marx, an assistant professor and epidemiologist at the Johns Hopkins Bloomberg School of Public Health, stressed the importance of vaccines for all ages, including kids.Rival Moderna hopes to be the first to offer vaccinations for the youngest children.
Last week, it filed with the Food and Drug Administration data it hopes will prove two of its low-dose shots work in children younger than 5.
Read more on fox29.com