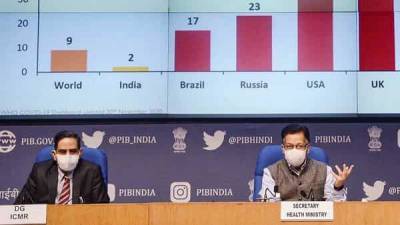
Government says serious adverse event will not delay launch of first covid-19 vaccine
Serum Institute of India’s Covishield trial.“The adverse event will not affect the timelines in any manner whatsoever," health secretary Rajesh Bhushan said on Tuesday.Serum Institute’s Covishield is widely expected to be available for immunisation of front health workers starting as early as January.On Saturday, the chief executive officer of the world’s largest vaccine manufacturer, Adar Poonawalla, said that the company plans to apply to the Drug Controller General of India for an emergency use license for the vaccine in about two weeks using the data from AstraZeneca’s phase 3 trial in the UK as well as the company’s own phase 2 and 3 bridging study in India.Covishield is originally developed by AstraZeneca and the University of.
Read more on livemint.com