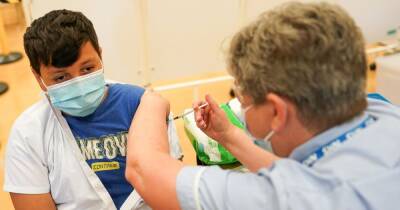
How Oxford- AstraZeneca covid-19 vaccine went from pole position to troubled start
LONDON : On June 5, researchers at the University of Oxford quietly made a change to a late-stage clinical trial of their COVID-19 vaccine.
In an amendment noted in a document marked CONFIDENTIAL, they said they were adding a new group of participants.The adjustment might seem minor in a large-scale study.
But it masked a mistake that would have potentially far-reaching consequences: Many of the United Kingdom trial subjects had inadvertently been given only about a half dose of the vaccine.Also Read | Inside the farmer disquiet at Delhi’s doorstepThe new volunteers would now receive the correct dose.
The trial continued.Much was riding on the Oxford vaccine, a British-led endeavour also involving UK drugs firm AstraZeneca.
Read more on livemint.com