In ‘milestone,’ FDA OKs simple, accurate coronavirus test that could cost just $5
Robert F. ServiceScience’s COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation.Coronavirus testing is set to get faster.
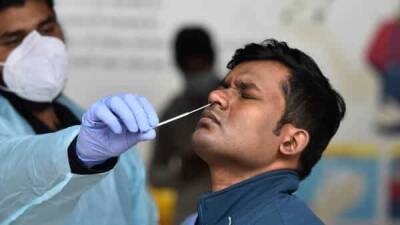
Yesterday, the U.S. Food and Drug Administration (FDA) gave an emergency use authorization to Abbott Laboratories for a 15-minute test that should ease bottlenecks.
Analogous to tests that detect HIV and influenza, the new diagnostic detects viral proteins, or antigens, that are unique to SARS-CoV-2, the virus that causes COVID-19.
Unlike conventional coronavirus diagnostics, Abbott’s test requires no specialized laboratory equipment. Abbott says the new assay will cost $5, and the company intends to produce tens of millions of the tests in September and 50 million in October.
Read more on sciencemag.org