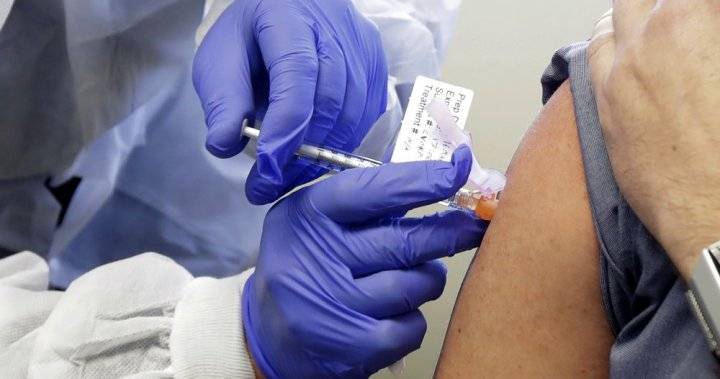
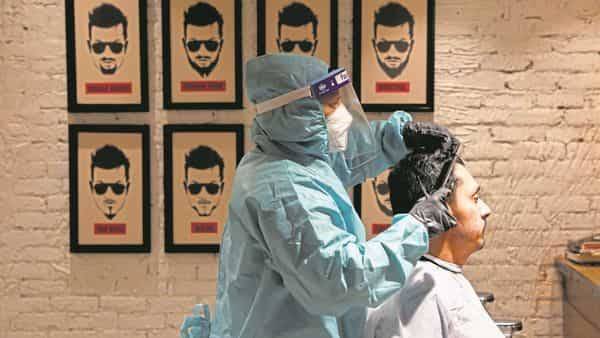
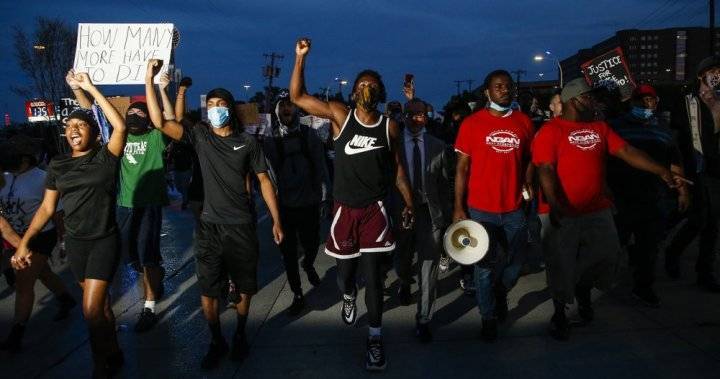5 days of remdesivir for COVID-19 may be enough
Results from an ongoing phase 3 study published yesterday in the New England Journal of Medicine showed no significant difference in the clinical status of hospitalized COVID-19 patients not requiring mechanical ventilation after a 5-day course of the antiviral drug remdesivir compared with patients who had a 10-day course.But the randomized, open-label trial lacked a placebo control, so the degree of benefit could not be determined.Sponsored by remdesivir maker Gilead, the trial took place at 55 hospitals in the United States, Asia, and Europe from Mar 6 to 26.
Investigators randomly assigned 397 coronavirus patients with an oxygen saturation of 94% or less and radiologic evidence of pneumonia to receive intravenous remdesivir, with a
Read more on cidrap.umn.edu