CDC panel recommends Pfizer COVID-19 vaccination
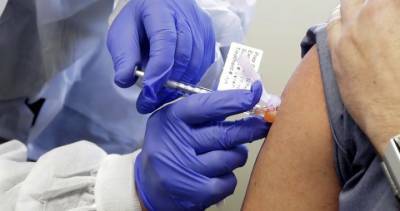
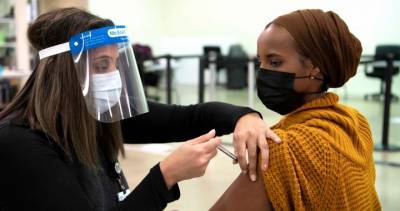
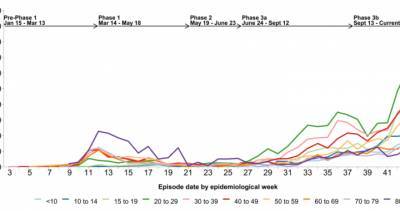
In an emergency session today, the vaccine advisory panel for the Centers for Disease Control and Prevention (CDC) voted to recommend that Americans receive the Pfizer-BioNTech COVID-19 vaccine, a key step that allows healthcare workers to begin administering the vaccine to people.The interim recommendation covers people ages 16 years and older under the Food and Drug Administration (FDA) emergency use authorization (EUA).The decision comes a day after the FDA issued its EUA, a step that cleared the way for the vaccine to begin shipping.The vote from the CDC's Advisory Committee on Immunization Practices (ACIP) passed with 11 votes in favor and 3 recusals.
Read more on cidrap.umn.edu