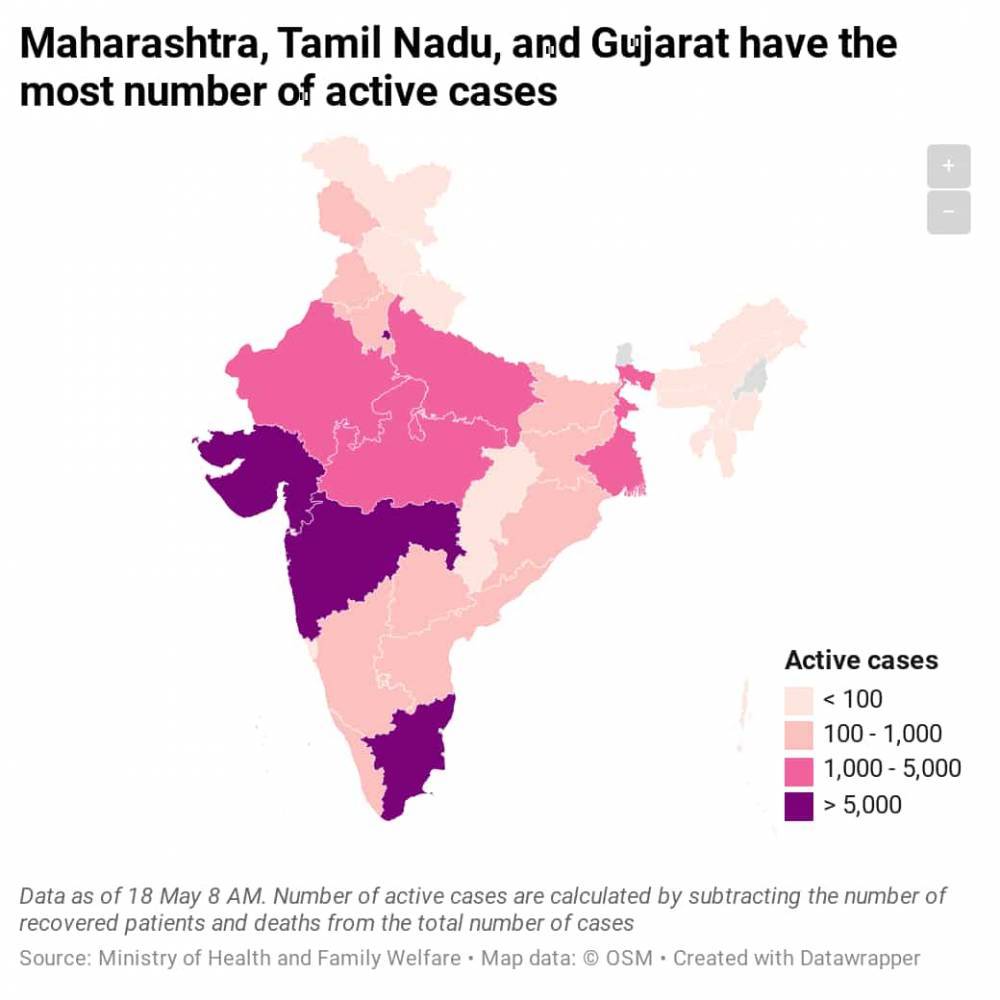
Covid-19: Glenmark begins phase 3 of clinical trials on antiviral Favipiravir
Glenmark Pharmaceuticals has initiated phase three of clinical trials in India on antiviral tablet Favipiravir, becoming the first company in the country to do so.
It had obtained approval from the Drug Controller General of India (DCGI) in late April. Favipiravir is a generic version of Avigan of Fujifilm Toyama Chemical Co Ltd in Japan, a subsidiary of Fujifilm Corporation. "Clinical trials have commenced and over 10 leading government and private hospitals in India are being enrolled for the study.
Glenmark estimates study completion by July or August," it said in a statement on Tuesday. Glenmark has successfully developed the active pharma ingredient (API) and the formulations for the product through its in-house R&D team.
Read more on livemint.com