FDA documents show Pfizer COVID vaccine protects after 1 dose
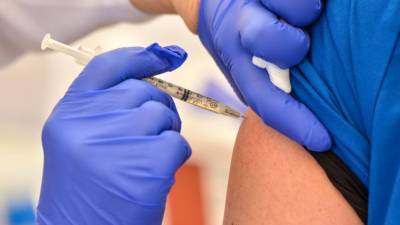
Food and Drug Administration (FDA) documents posted in advance of advisory group consideration of emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine revealed promising new findings, including strong protection after the first dose and protection in groups at risk for disease complications.The good news comes on the same day immunization with the vaccine began with much fanfare in the United Kingdom, where a 90-year-old woman who lives in Coventry was the first to receive it outside of a vaccine trial.Additional phase 3 findingsThe FDA analysis is based on detailed data that weren't spelled out in Pfizer's press release last month that described its initial efficacy findings.
Read more on cidrap.umn.edu