FDA revokes authorisation for antimalarials to treat Covid-19
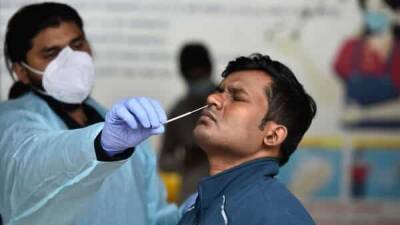
Sign up here for GlobalData's free bi-weekly Covid-19 report on the latest information your industry needs to know.The US Food and Drug Administration (FDA) has revoked the emergency use authorisation (EUA) for antimalarials, chloroquine phosphate and hydroxychloroquine sulfate, to treat some hospitalised Covid-19 patients.
In March, FDA awarded the EUA for the use of these drugs donated to the Strategic National Stockpile when a clinical trial was not available or trial participation was not feasible.
According to the regulatory agency, chloroquine and hydroxychloroquine no longer meet the legal criteria for EUA. Data from an ongoing analysis of the EUA and scientific research showed that the drugs are not likely to be effective against.
Read more on pharmaceutical-technology.com