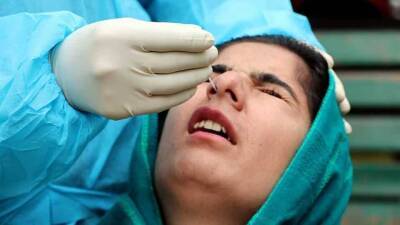
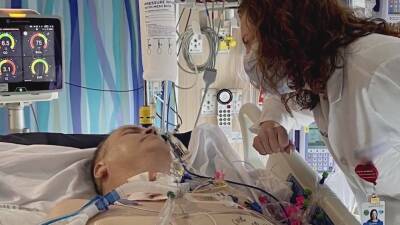
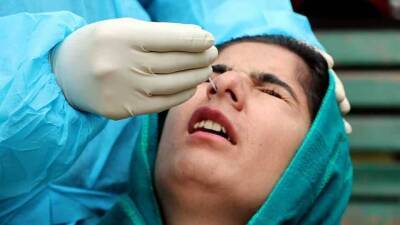
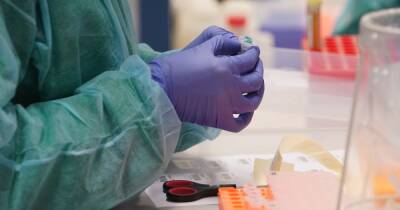
Oxford University Covid vaccine test stopped as volunteer has 'serious adverse reaction'
Coronavirus vaccine has announced it has suspended testing of the potential medicine to conduct a "review of safety data" after a volunteer suffered a negative reaction to the drug.A spokesperson for AstraZeneca, a company working with Oxford University to create a potential vaccine, said the company’s “standard review process triggered a pause to vaccination to allow review of safety data.” No firmer information has yet been released on the nature of the reaction, or when it happened, although a source reportedly claimed the participant in question is expected to make a full recovery.The spokesperson explained the pause is "a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while.
Read more on dailystar.co.uk