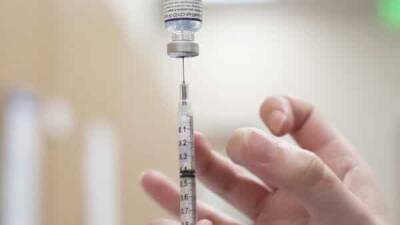
Pfizer Reveals Their Coronavirus Vaccine Is 95% Effective After Completing Third Study Trial
A new report from Pfizer has come out of their coronavirus vaccine trials about the success rate of the vaccine they have been developing to help eradicate the pandemic.
The company has submitted a request to the Food and Drug Administration for emergency approval, as data for their vaccine has proven 95 percent effective.
Just a few weeks earlier, the company, along with collaborator BioNTech, announced that preliminary results from trials showed its potential vaccine was at least 90 percent effective.
Now, after completing a phase three study, the vaccine has proven to be 95 percent effective with “no serious safety concerns observed” in the trial participants. “Our work to deliver a safe and effective vaccine has never been more urgent,
Read more on justjared.com