U.S. FDA warns against using malaria drug on COVID-19 patients outside hospitals
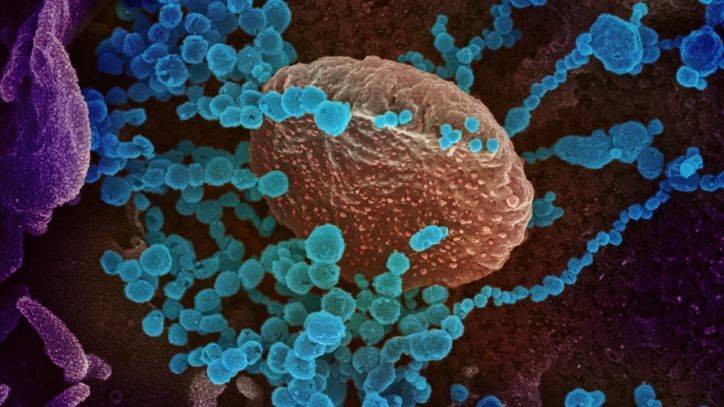
The U.S. Food and Drug Administration on Friday cautioned against the use of malaria drugs, hydroxychloroquine and chloroquine, in COVID-19 patients outside of hospitals and clinical trials, citing risks of serious heart rhythm problems.
The agency’s announcement comes a day after the European Union’s drug regulator warned of the drugs’ side effects and urged medical professionals to closely monitor patients on the medicines.
The FDA said it was aware of increased use of these medicines through outpatient prescriptions and the drugs could cause abnormal heart rhythms and dangerously rapid heart rate.
Decades-old hydroxychloroquine has been touted by U.S. President Donald Trump as a “game changer” in the fight against the novel coronavirus
Read more on globalnews.ca