US slashes testing rules to speedup coronavirus screening
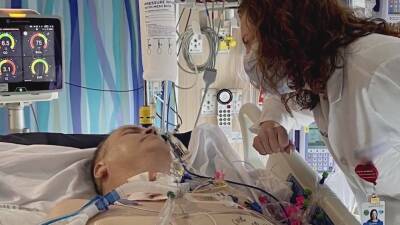
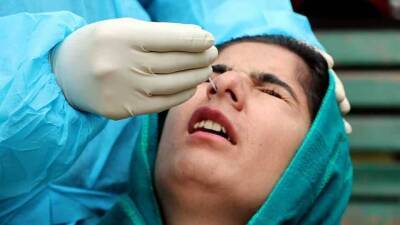
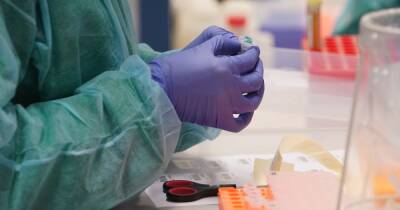
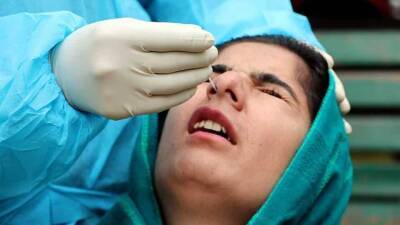
WASHINGTON, D.C. – The Trump administration is slashing regulations governing test development in a bid to ramp up screening for the coronavirus amid nationwide frustration with the slow pace of the effort.
The unprecedented steps by the Food and Drug Administration could boost testing capacity at some U.S. labs, but also complicate efforts to assure the accuracy of tests and track who receives them.
More than eight weeks after the first U.S. case of COVID-19 was detected, the U.S. still struggles to conduct mass screening and provide definitive figures on the number of people tested.
Late Monday, the FDA said state health departments could essentially begin self-regulating and approving tests developed by their government-run laboratories.
Read more on clickorlando.com