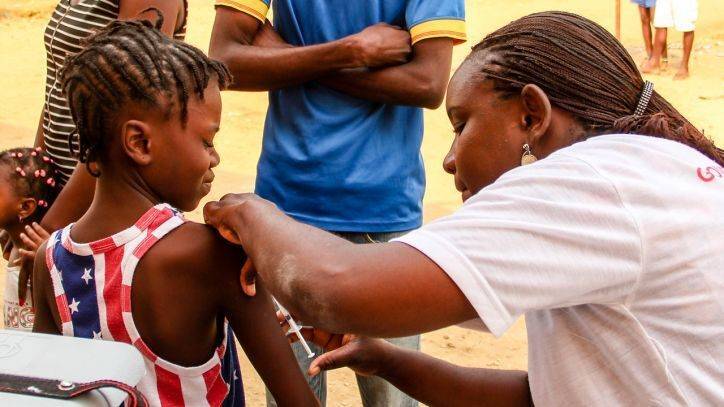US COVID-19 deaths pass 90,000 as Moderna vaccine delivers promising phase 1 results
Moderna's COVID-19 vaccine candidate appears safe for human use, the company said today as it released results from the phase 1 trial of mRNA-1273.The dose-dependent trial sets the stage for a phase 2 trial, expected to start shortly and a larger phase 3 trial which will begin in July.
The phase 1 trail showed an immune response in a small number healthy adult participants (8), who produced neutralizing antibodies to the virus that were similar to the antibodies seen in convalescent COVID-19 patient's plasma."With today's positive interim phase 1 data and the positive data in the mouse challenge model, the Moderna team continues to focus on moving as fast as safely possible to start our pivotal phase 3 study in July and, if successful, file
Read more on cidrap.umn.edu