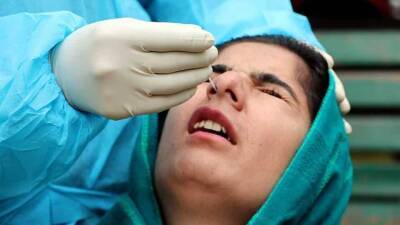
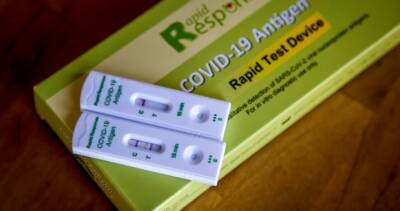
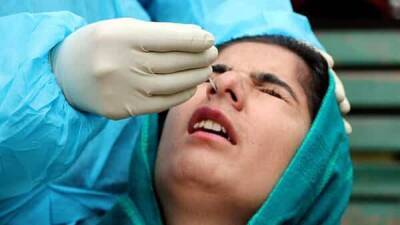
COVID-19 Testing at Laboratories: Questions and Answers
CDC provides the test kits for public health laboratories (PHLs) to perform real-time RT-polymerase chain reaction (rRT-PCR) detection of the SARS-CoV-2 virus (the virus that causes COVID-19) in respiratory specimens.
CDC received Emergency Use Authorization (EUA) from the Food and Drug Administration (FDA) on February 4, 2020 for use of this rRT-PCR test to detect the virus in upper and lower respiratory specimens.
These test kits are available through the International Reagent Resource (IRR)external iconexternal icon. For over ten years, CDC has provided test kits and reagents to PHLs through the IRR.
This resource was established to support state and local public health laboratories, Department of Defense laboratories, and other.
Read more on cdc.gov