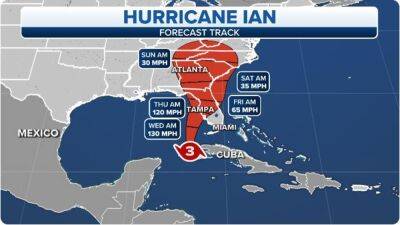
FDA authorizes updated COVID boosters from Moderna and Pfizer
The Food and Drug Administration (FDA) today announced that it has authorized for emergency use bivalent booster shots that target the Omicron BA.4/BA.5 subvariants, setting the stage for Centers for Disease Control and Prevention (CDC) advisory group recommendation discussions tomorrow.In other developments, the World Health Organization (WHO) said cases and deaths declined last week, while warning that new surges could occur in the months ahead.Another step toward September booster rolloutIn a statement, the FDA said the authorizations cover the Moderna bivalent vaccine in those ages 18 and older, and the Pfizer/BioNTech vaccine covers people ages 12 and older.
The updated boosters can be given at least 2 months after primary vaccination or the last booster vaccination.BA.4 and BA.5 Omicron subvariants are still circulating widely in the United States, and health officials predict that they will remain through the fall and winter.Robert Califf, MD, FDA commissioner, said, "As we head into fall and begin to spend more time indoors, we strongly encourage anyone who is eligible to consider receiving a booster dose with a bivalent COVID-19 vaccine to provide better protection against currently circulating variants."Also as part of today's announcement, the FDA pulled its authorization of monovalent boosters for most groups, except Pfizer for kids ages 5 through 11 years.
However, the vaccines are still authorized for the primary series in those ages 6 months and older.When the two companies applied for emergency use of the updated boosters, they said they had already scaled up manufacturing to be ready to deliver doses in September, if authorized.The FDA based its authorization on clinical data from trials for a bivalent
Read more on cidrap.umn.edu